HIGH DIAGNOSTIC ACCURACY
ThyroSeq® is the most accurate test
for thyroid nodules and cancer
- Comprehensive genomic profiling of thyroid nodules using next-generation DNA and RNA sequencing
- Clinically validated in the largest prospective, double-blind, multicenter study reported in JAMA Oncology1 and supported by multiple independent studies2-8
- Highest NPV and PPV among well-validated tests 1
- Reliable diagnosis of all types of thyroid nodules, including Hürthle cell nodules, medullary thyroid carcinoma, parathyroid, or other non-thyroidal lesions in a single workflow
Has already helped inform clinical management for > 70,000 patients9
94%
Sensitivity
82%
Specificity
97%
NPV
66%
PPV
Test performance according to the largest multicenter clinical validation study, reported in JAMA Oncology
PREVENTION OF UNNECESSARY SURGERIES
High reduction of diagnostic surgeries
in nodules with indeterminate cytology
With very low (3%) residual cancer risk in ThyroSeq-negative nodules, surgery can be avoided in patients with Bethesda III-IV cytology nodules based on NCCN and ATA guidelines
- University of California Los Angeles, CA2
- Duke University, NC3
- Boston Medical Center, MA4
- McGill University, Quebec, Canada5
- New York University, NY 6
- University of Pennsylvania, PA7
PERSONALIZED MANAGEMENT OF CANCEROUS NODULES
ThyroSeq® uniquely reports the probability of cancer
and a prediction of cancer recurrence,
informing personalized patient management
Prediction of cancer recurrence is based on:
-
Comprehensive genomic profiling of DNA and RNA to detect four main classes of molecular alterations 1,10
- Inclusion of all markers of aggressive thyroid cancer (TERT, TP53, AKT1, PIK3CA, and others) 1,10
- Unique and proprietary database of >3,000 thyroid nodules with known surgical outcome9
Specific management recommendations included in each ThyroSeq® report:
-
Patients with a low-risk for recurrence profile detected by ThyroSeq® may be candidates for limited thyroid surgery (lobectomy)
-
Patients with a high-risk for recurrence profile may be candidates for total thyroidectomy and radioactive iodine treatment
-
Patients with advanced thyroid cancer may benefit from ThyroSeq® detection of therapeutic targets (BRAF, NTRK, RET, ALK) linked to FDA approved drugs and clinical trials
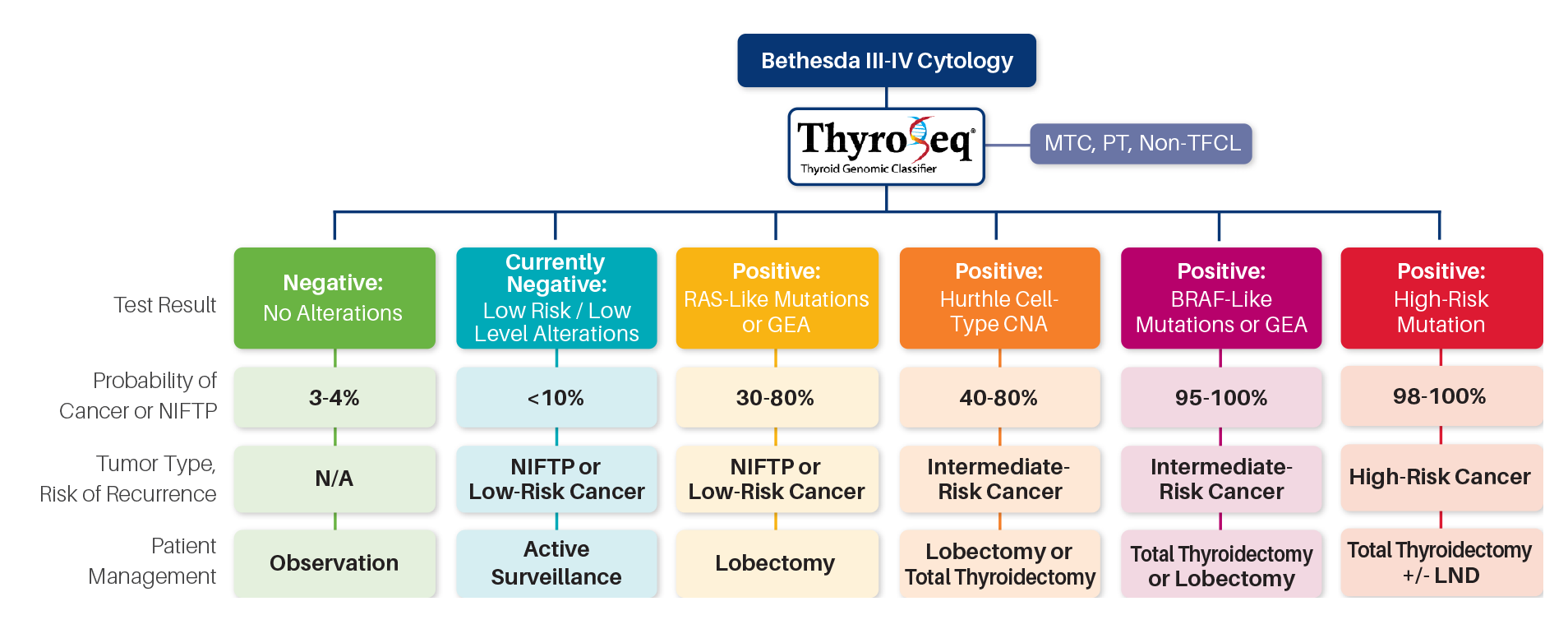
Abbreviations: MTC, medullary thyroid cancer; PT, parathyroid; Non-TFCL, non-thyroid follicular cell lesion; GEA, gene expression alterations; CNA, copy number alterations; LND, lymph node dissection.
FLEXIBILITY IN SAMPLE TYPES
ThyroSeq® testing has been validated for use
on a variety of specimen types:
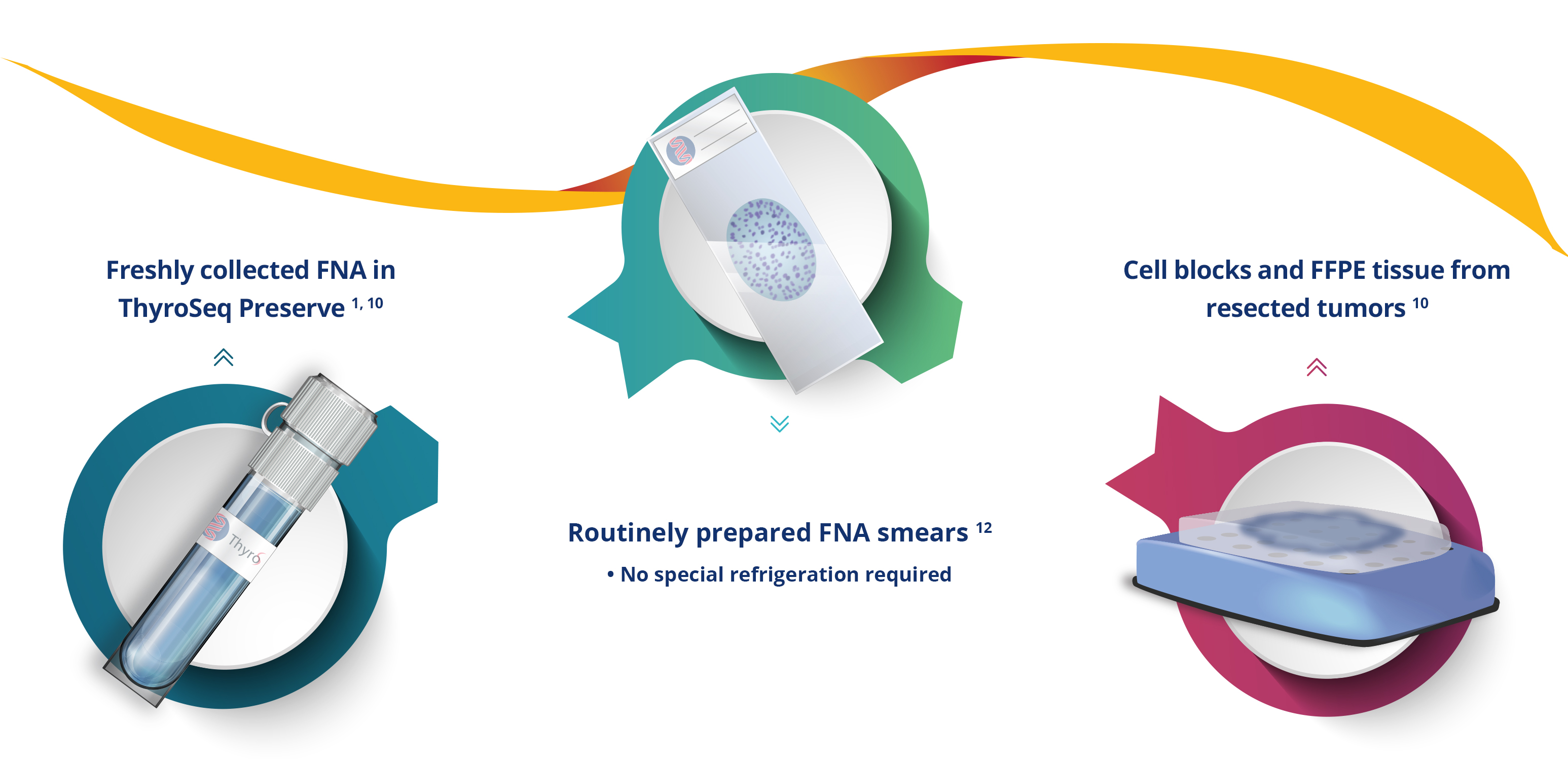
High success rate of testing: 97-100% for fresh FNA samples,
81% for smears (PAP, Diff-Quik) 3-5, 10-12
References:
1.Steward DL, et al. JAMA Oncol. 2018. 2.Chin PD, et al. Endocrin Pathol. 2020. 3.Jug R, et al. Cancer Cytopathol. 2020. 4.Guan H, et al. Thyroid. 2020. 5.Chen T, et al. Thyroid. 2020. 6.Schatz-Siemers N, et al. Diagn Cytopathol. 2019. 7.Desai D, et al. Cancer Cytopathol. 2020. 8.Zhu CY, et al. Thyroid. 2020. 9.UPMC, data on file. 10.Nikiforova MN, et al. Cancer. 2018. 11.Carty SE, et al. Ann Surg. 2020 12.Nikiforova MN, et al. Cancer Cytopathol. 2020.
ThyroSeq Molecular Tumor Board
THYROSEQ® EDUCATIONAL RESOURCES
Sign up to receive information
on our next virtual thyroid tumor board and other educational resources
Sign up is required for password access to ThyroSeq Videos
Molecular Profiling of 50 734 Bethesda III-VI Thyroid Nodules by ThyroSeq v3: Implications for Personalized Management
Comprehensive genomic analysis of thyroid nodules for multiple classes of molecular alterations detected in a large series of fine needle aspiration (FNA) samples has not been reported.

Thyroid Cytology Smear Slides: An Untapped Resource for ThyroSeq® Testing
Molecular testing of thyroid nodules with indeterminate fine-needle aspiration (FNA) cytology is commonly used to guide patient management and is typically performed on freshly collected FNA samples. In this study, the authors evaluated the performance of the ThyroSeq® test in cytology smear slides.
Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study
In this prospective, blinded, multicenter study, ThyroSeq® GC demonstrated a high sensitivity/NPV and reasonably high specificity/PPV, which may obviate diagnostic surgery in up to 61% of patients with Bethesda III-IV indeterminate nodules, and up to 82% of all benign nodules with indeterminate cytology. Information on specific genetic alterations obtained from FNA may help inform individualized treatment of patients with a positive test result.
Learn about ThyroSeq®
Dr. Steven P. Hodak, Professor of Medicine and Endocrinology at NYU School of Medicine and Director of Endocrinology, NYU Langone Medical Center, Tisch Hospital, discusses the use of ThyroSeq® test for thyroid nodules with indeterminate cytology.
Get the latest updates, case studies, abstracts, and information on ThyroSeq testing
Test Ordering
We provide physicians with several options for ordering the ThyroSeq test for patients with thyroid nodules.
Resources
View various ThyroSeq publications, presentations, practice guidelines, news, and more.
Contact Us
Are you interested in learning more about ThyroSeq or ordering the ThyroSeq test to more accurately diagnose thyroid nodules?